Matthew Faber

Graduate Student, Materials Chemistry
Department of Chemistry
University of Wisconsin-Madison
Biographical Sketch
B.S., The University of Texas at Dallas, Chemistry, 2008
Ph.D., University of Wisconsin–Madison, Materials Chemistry, 2009–Present
Research
My research involves the investigation of alternative, earth-abundant materials toward simultaneously improving the performance and lowering the cost of photovoltaic solar cells. Several materials are currently under investigation as improved active components (e.g., as photon absorbers or charge transporters) in a number of photovoltaic cell designs, including liquid-junction, solid-state, and hybrid devices.
Recently we reported the synthesis of metallic cobalt pyrite (cobalt disulfide, CoS2; cattierite) thin films on glass via the facile thermal sulfidation of a cobalt precursor film and demonstrated their integration into liquid-junction CdS/CdSe quantum dot-sensitized solar cells (QDSSCs) as the counter electrode (Figures 1a and 1b, inset). In these QDSSCs, the aqueous sulfide/polysulfide (S2–/ Sn2–) electrolyte is commonly used as the hole-transporting medium; however, sulfur species in this electrolyte are known to poison traditional platinum counter electrodes and reduce their electrocatalytic activity toward polysulfide reduction, reducing QDSSC performance. By replacing the platinum counter electrode of a QDSSC with a CoS2 thin film counter electrode, we observed a 54 (±14)% increase in solar light-to-electricity conversion efficiency, with conversion efficiencies as high as 4.16% achieved. Various electrochemical characterizations were used to rationalize the improved performance of the CoS2 counter electrode. We found that not only did the CoS2 electrode exhibit a lower charge transfer resistance toward S2–/ Sn2– than platinum electrodes, it was also less susceptible to poisoning, thereby enabling stable and reproducible performance (Figure 1c).
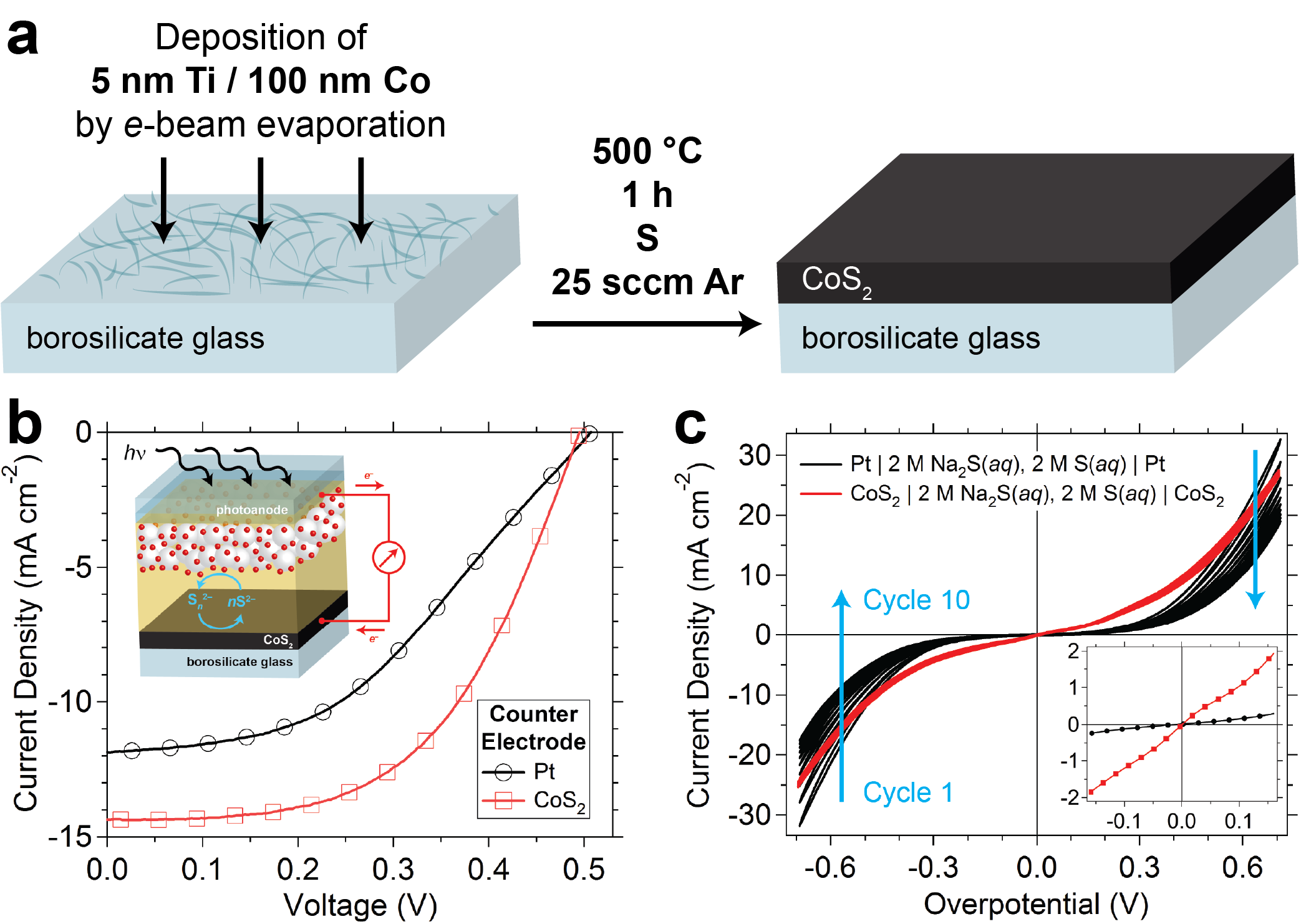
Figure 1.(a) Schematic depiction of the preparation of a cobalt pyrite (CoS2) film electrode via the thermal sulfidation of a 100 nm thick cobalt film deposited over a titanium adhesion layer on a roughened borosilicate glass substrate by electron-beam evaporation. (b) Typical current density-voltage (J–V) curve demonstrating the improved performance of quantum dot-sensitized solar cells (QDSSCs) assembled with a CoS2 counter electrode (inset). (c) Cyclic voltammetric (CV) characterization of CoS2 on glass and Pt on FTO/glass electrodes in symmetrical sandwich-style thin-layer electrochemical cells filled with an aqueous sulfide/polysulfide (2 M S2−/2 M S) electrolyte solution. Ten complete CV cycles show the rapid deactivation of the Pt electrode surface due to sulfur species adsorption, which results in diminishing current density upon repeated cycling. The inset shows the linear (i.e., low overpotential) region of the tenth anodic voltage scan for each cell, which highlights the low polarization resistance of the CoS2 electrode relative to that of the Pt electrode, indicating the greater electrocatalytic activity of CoS2 toward both oxidation and reduction of the sulfide/polysulfide redox couple.
We are continuing our investigation of alternative materials and designs that enable the improved performance of low-cost photovoltaic solar cells.
Publications
1. Faber, M. S.; Park, K.; Cabán-Acevedo, M.; Santra, P. K.; Jin, S. Earth-Abundant Cobalt Pyrite (CoS2) Thin Film on Glass as a Robust, High-Performance Counter Electrode for Quantum Dot-Sensitized Solar Cells. J. Phys. Chem. Lett. 2013, 4, 1843–1849.
2. Selinsky, R. S.; Ding, Q.; Faber, M. S.; Wright, J. C.; Jin, S. Quantum Dot Nanoscale Heterostructures for Solar Energy Conversion. Chem. Soc. Rev. 2013, 42, 2963–2985.
3. Cabán-Acevedo, M.; Faber, M. S.; Tan, Y.; Hamers, R. J.; Jin, S. Synthesis and Properties of Semiconducting Iron Pyrite (FeS2) Nanowires. Nano Lett. 2012, 12, 1977–1982.
4. Yan, C.; Higgins, J. M.; Faber, M. S.; Lee, P. S.; Jin, S. Spontaneous Growth and Phase Transformation of Highly Conductive Nickel Germanide Nanowires. ACS Nano 2011, 5, 5006–5014.
