Electrocatalytic and Photoelectrochemical Conversion of Energy and Chemicals Using Earth-Abundant Nanomaterials
Electrocatalysis is at the heart of electrolyzers, fuel cells, and other renewable energy conversion and storage technologies, which provide sustainable pathways for the production of fuels and chemicals in cleaner and more reliable and environmentally friendly ways. We develop efficient and inexpensive new earth-abundant (nanostructured) electrocatalysts for hydrogen evolution reaction (HER), oxygen evolution reaction (OER), and selective electrochemical production of hydrogen peroxide (H2O2), and other value-added chemicals. We have also integrated the highly active earth-abundant HER electrocatalysts we have established into photocathodes for direct solar-driven photoelectrochemical (PEC) hydrogen fuel generation.

Hydrogen Evolution Reaction (HER)
We have discovered several new families of earth-abundant electrocatalysts for hydrogen evolution reaction (HER) and significantly enhanced their catalytic activity by controlling the nanostructures, polymorphs, and defects.1,2 Layered transition-metal dichalcogenides is a family of two-dimensional (2D) materials with a general formula of MX2 (for examples, M = Mo, W; X = S, Se). We initially discovered dramatically enhanced HER catalysis from chemically exfoliated metallic nanosheets of 1T-MoS2 and WS2, relative to their semiconducting 2H polymorphs.3,4 Besides tuning of the crystal phase and electronic structures, we further revealed that vacancies, edges, and disorders also contribute significantly to the HER catalytic activity of MoS2 and MoSe2.5,6

Figure 1. Enhanced HER catalysis using chemically exfoliated metallic nanosheets of 1T-MoS2.3
We also enhanced the HER catalytic activity of pyrite-phase transition-metal dichalcogenides (M = Fe, Co, Ni; X = S, Se) by controlling the nanostructures7 and tuning the metal and non-metal alloy compositions.8,9 We further designed, discovered, and established ternary pyrite-type cobalt phosphosulfide (CoPS) as one of the most efficient earth-abundant catalysts in acidic conditions that do not contain expensive noble metals.10 Nanostructured CoPS can achieve HER catalytic activity very close to that of platinum with outstanding long-term operation stability. In collaboration with Prof. JR Schmidt, we also studied the facet-dependent HER catalytic activity of CoPS single crystals both experimentally and theoretically.11 Further tuning the chemical compositions and electronic structures of these transition metal compounds led to increasingly complex HER catalysts, such as a highly active quaternary NiFePS3 catalyst.12

Figure 2. Enhanced HER catalysis using earth-abundant ternary pyrite-type cobalt phosphosulfide (CoPS).10
Oxygen Evolution Reaction (OER)
Our work on earth-abundant oxygen evolution reaction (OER) electrocatalysts has been focused on developing and understanding transition-metal layered double hydroxides (LDHs), which are a family of 2D materials that consist of positively charged brucite-like MII(OH)2 layers (with MII cations partially substituted by MIII cations) and charge-balancing anions between them. LDHs provide much compositional and structural diversity and tunability of electrochemical properties. In our earlier work, well-defined NiCo LDH nanoplates were synthesized by carefully controlling the precursor supersaturation using a high temperature high pressure hydrothermal continuous flow reactor (HCFR) we developed, and then exfoliated into thinner nanosheets that show enhanced OER catalytic activity.13 We also demonstrated a chemical etching strategy to convert NiGa LDH nanoplates into porous β-Ni(OH)2 nanosheets with enhanced OER catalytic performance and other nickel-containing electrocatalysts.14 To investigate how the interlayer anions and spacings influence the OER catalytic activity of NiFe LDHs, we found carbonate, chloride, and sulfate-intercalated NiFe LDHs display similar OER catalytic performance and undergo anion exchange to carbonate in alkaline electrolyte under ambient conditions; in contrast, dodecyl sulfate-intercalated NiFe LDHs prepared by anion exchange show enhanced OER catalytic performance unexplained by surface area and resist carbonate exchange during electrochemical cycling.15

Figure 3. (a) OER electrocatalysis using exfoliated NiCo layered double hydroxides (LDHs).13 (b) Influence of interlayer anions on OER catalytic activity of NiFe LDHs.15
NiFe LDHs are arguably the most active OER electrocatalysts among bimetallic transition metal LDHs, but the origin of their high OER catalytic activity and exact mechanistic details remain elusive or controversial. In an effort to gain insights into the role of Fe in NiFe LDHs, we helped Prof. Shannon Stahl’s group to use operando Mössbauer spectroscopy to provide direct evidence for the formation of Fe4+ in NiFe LDH catalysts under steady-state OER conditions with important mechanistic implications.16 Inspired by the observation of high-valence-state Fe4+ in NiFe LDHs under OER conditions, we hypothesized the OER catalytic activity of NiFe LDHs could be further enhanced by incorporating a third metal that can readily adopt high valence states, and discovered trimetallic NiFeCr LDHs with even higher intrinsic OER catalytic activity than NiFe LDHs.17 The stability of NiFeCr LDHs under OER conditions were confirmed using X-ray photoelectron spectroscopy (XPS), electron paramagnetic resonance spectroscopy (EPR), and elemental analysis. We are further investigating the catalytic mechanisms of these complex multimetallic OER catalysts.

Figure 4. (a) Operando Mössbauer spectroscopic detection of Fe4+ in NiFe LDHs under OER conditions.16 (b) Highly active trimetallic NiFeCr LDHs as OER catalysts.17
Selective Electrocatalytic Production of Hydrogen Peroxide (H2O2)
Electrocatalytic production of fuels and value-added chemicals beyond hydrogen has the potential to transform the chemical industry since the electricity from renewable sources such as solar and wind energy has become increasingly affordable and available. A major challenge is to develop earth-abundant electrocatalysts that can produce targeted value-added chemicals with high selectivity and activity, yet are still robust and inexpensive. We are working on developing earth-abundant catalysts that are selective toward two-electron oxygen reduction reaction (2e- ORR) for the de-centralized electrochemical production of hydrogen peroxide (H2O2).
Commercial production of H2O2 has been almost exclusively through an indirect anthraquinone process, which (1) uses large quantities of H2 gas (both costly and energy intensive to obtain); (2) requires long-distance transportation of concentrated H2O2 to end-users with significant cost and safety concerns. Direct H2O2 production via electrochemical, rather than chemical, reduction of O2 eliminates the need for H2 gas, allowing for not only reduction in both costs and energy consumption but also safer deployment in a decentralized fashion. In collaboration with Prof. JR Schmidt, we are developing new earth-abundant ORR electrocatalysts that can selectively reduce O2 to H2O2 via the two-electron pathway (rather than the four-electron pathway to water). Our recent joint computational/experimental study demonstrated that cobalt pyrite (CoS2) is an earth-abundant transitional-metal compound that is active and selective toward 2e- ORR in acidic and noncorrosive neutral solution.18 More importantly, this discovery opens up a new direction and the general mechanistic insights gained from this study will guide our exploration of diverse classes of earth-abundant transition-metal compounds in search of more active and selective electrocatalysts for efficient decentralized H2O2 production.
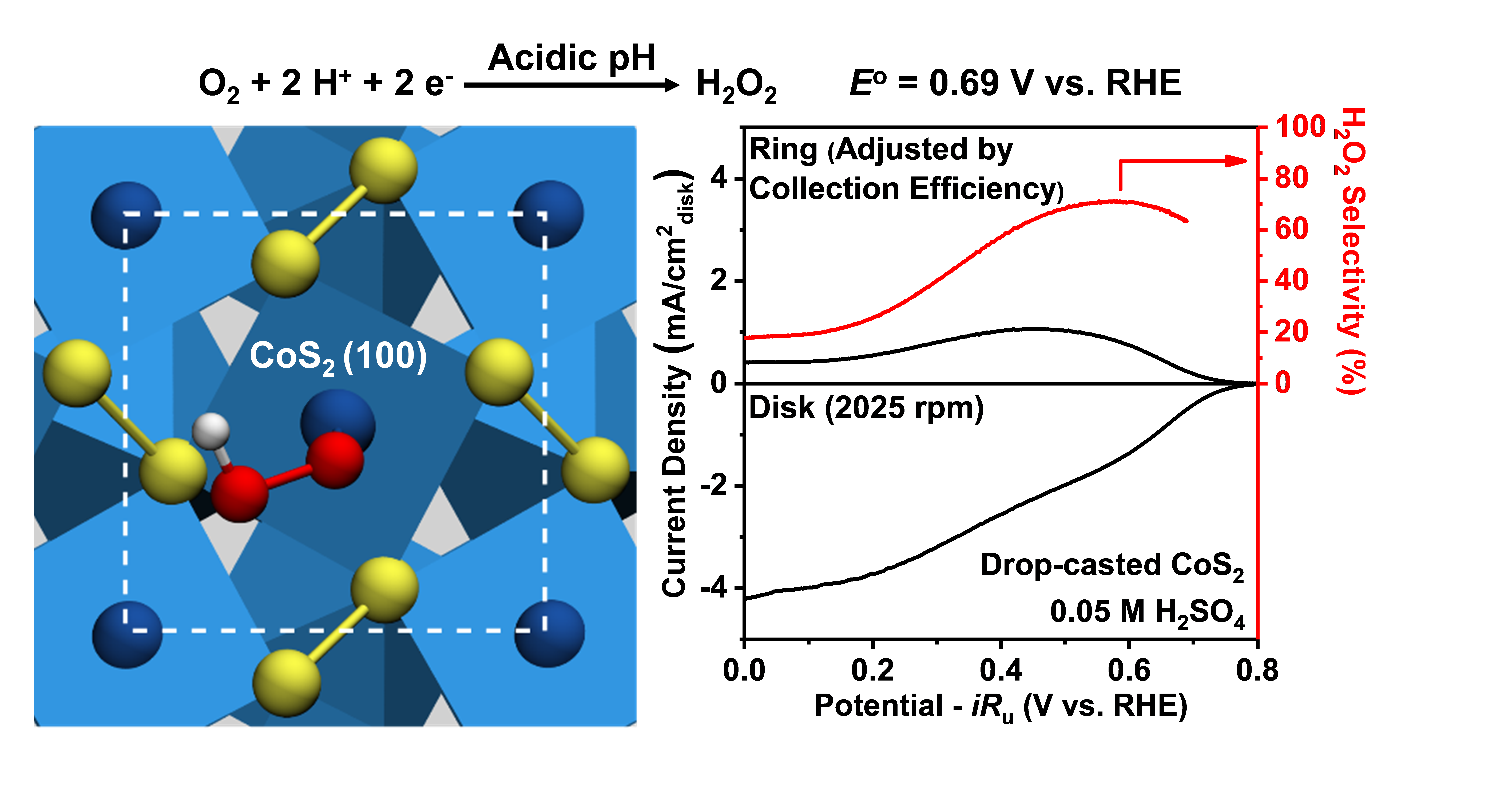
Figure 5. Electrocatalytic production of H2O2 by selective two-electron oxygen reduction using earth-abundant cobalt pyrite (CoS2).18
In addition, electrochemical oxidation of biomass-derived platform molecules into value-added oxygenated commodity chemicals is a promising alternative to producing these industrially important building block organic molecules from fossil fuels. However, few efficient, robust, and inexpensive electrocatalysts are available for such electrochemical transformation. We collaborated with Prof. George Huber’s group to demonstrate NiFe LDHs can efficiently and selectively catalyze the electrochemical oxidation of 5-hydroxymethylfurfural (HMF) to 2,5-furandicarboxylic acid (FDCA) in alkaline solution with a high Faradaic efficiency and a high yield of FDCA.19 We are investigating a broader range of earth-abundant electrocatalysts for selective oxidation of other biomass-derived platform molecules with more complicated reaction pathways.
Photoelectrochemical Hydrogen Fuel Generation
Generating hydrogen fuel through solar-driven photoelectrochemical (PEC) water splitting is a promising approach to providing affordable clean energy, reducing our reliance on fossil fuels, and mitigating the impact of climate change. The sustainable production of hydrogen demands efficient and robust earth-abundant catalysts that are not based on platinum and other precious metals for HER catalysis. Efficient PEC hydrogen generation systems that integrate the earth-abundant electrocatalysts that we have established with high performance semiconductor photoelectrode materials (such as those based on silicon) have been rationally designed and demonstrated, often in collaboration with Prof. Jr-Hau He’s group. Specific examples include the integration of silicon micropyramid photocathode with chemically exfoliated MoS2,20 MoQxCly (Q = S, Se),21 and CoPS electrocatalyst.10 Through these work, we have learned the rational design principles for integrating active electrocatalysts with photoelectrodes for achieving high performance integrated PEC systems.2
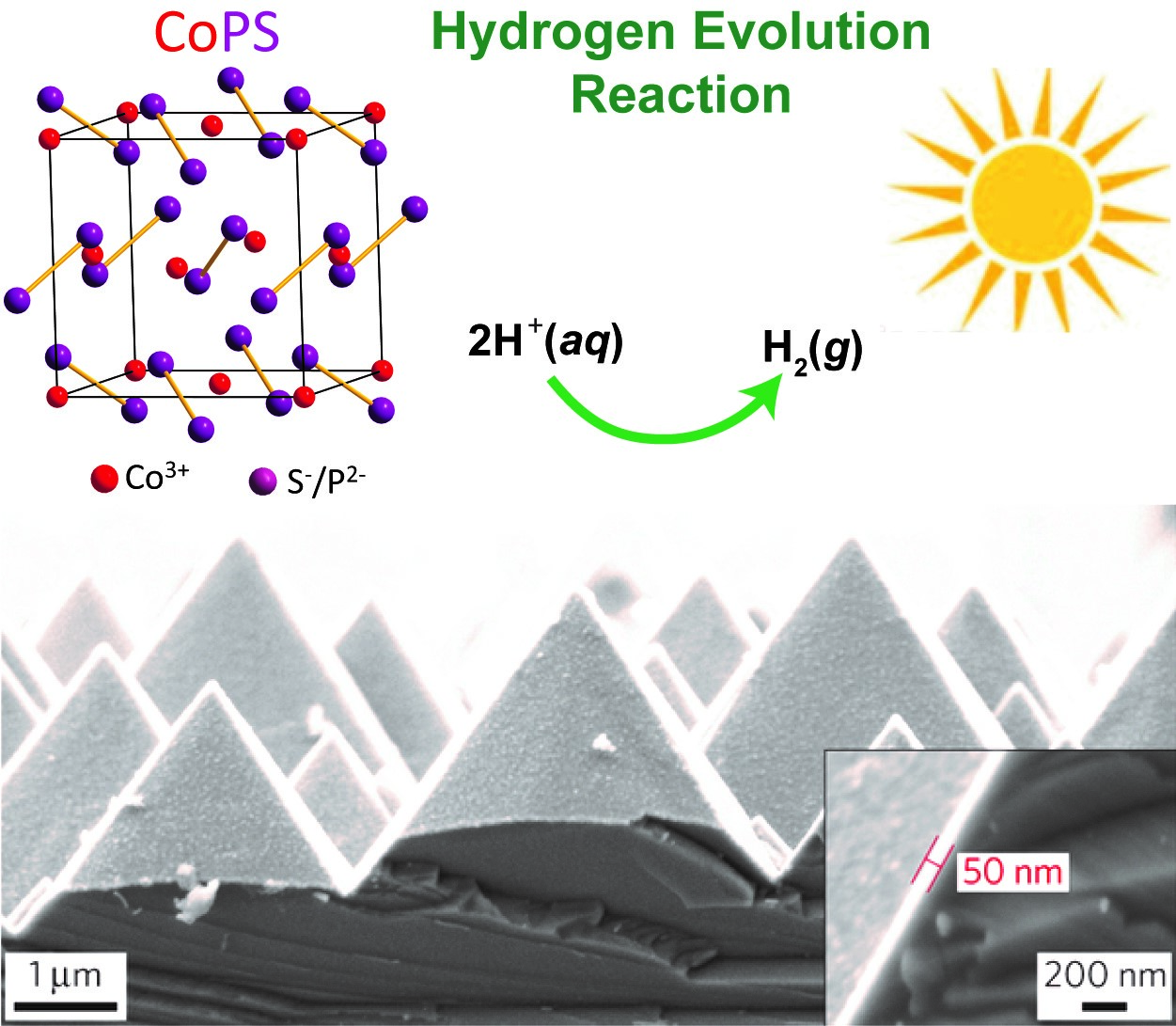
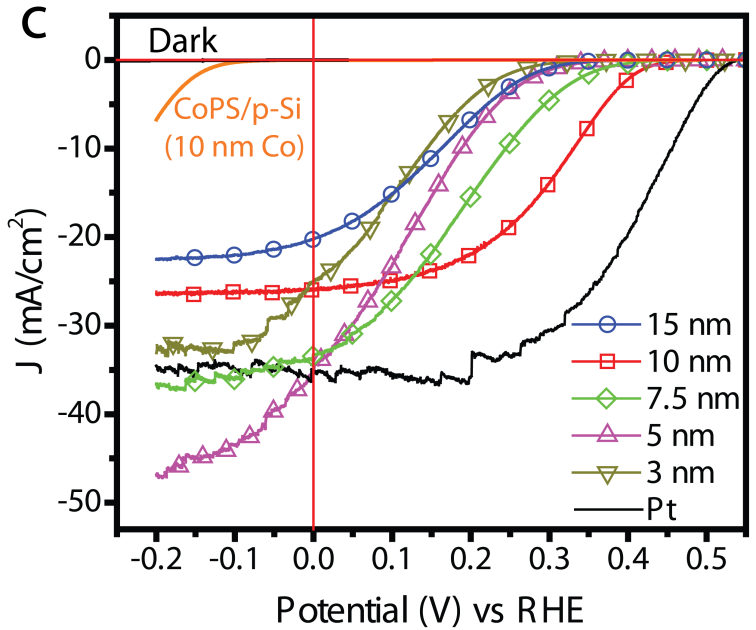
Figure 6. Photoelectrochemical hydrogen fuel generation using integrated CoPS/Si micropyramid photocathode.10
References
1. Matthew S. Faber and Song Jin; Earth-abundant inorganic electrocatalysts and their nanostructures for energy conversion applications, Energy. Environ. Sci., 2014, 7, pp 3519-3542, DOI: 10.1039/C4EE01760A
2. Qi Ding, Bo Song, Ping Xu, Song Jin, Efficient Electrocatalytic and Photoelectrochemical Hydrogen Generation Using MoS2 and Related Compounds, Chem, 1(5), pp.699-726., DOI: 10.1016/j.chempr.2016.10.007
3. Mark A. Lukowski, Andrew S. Daniel, Fei Meng, Audrey Forticaux, Linsen Li, and Song Jin; Enhanced Hydrogen Evolution Catalysis from Chemically Exfoliated Metallic MoS2 Nanosheets J. Am. Chem. Soc. 2013, 135 (28), pp 10274–10277.
4. Mark A. Lukowski, Andrew S. Daniel, Caroline R. English, Fei Meng, Audrey Forticaux, Robert Hamers, and Song Jin; Highly Active Hydrogen Evolution Catalysis from Metallic WS2 Nanosheets, Energy Environ. Sci., 2014, 7, pp 2608-2613, DOI: 10.1039/C4EE01329H
5. Ying Yin, Jiecai Han, Yumin Zhang, Xinghong Zhang, Ping Xu, Quan Yuan, Leith Samad, Xianjie Wang, Yi Wang, Zhihua Zhang, Peng Zhang, Xingzhong Cao, Bo Song, and Song Jin, Contributions of Phase, Sulfur Vacancies, and Edges to the Hydrogen Evolution Reaction Catalytic Activity of Porous Molybdenum Disulfide Nanosheets, J. Am. Chem. Soc., 2016, 138 (25),pp 7965–7972, DOI: 10.1021/jacs.6b03714
6. Ying Yin, Yumin Zhang, Tangling Gao, Tai Yao, Xinghong Zhang, Jiecai Han, Xianjie Wang, Zhihua Zhang, Ping Xu, Peng Zhang, Xingzhong Cao, Bo Song and Song Jin, Synergistic Phase and Disorder Engineering in 1T-MoSe2 Nanosheets for Enhanced Hydrogen-Evolution Reaction, Adv. Mater.,(2017), DOI: 10.1002/adma.201700311
7. Matthew S. Faber, Rafal Dziedzic, Mark A. Lukowski, Nicholas S. Kaiser, Qi Ding, and Song Jin; High-Performance Electrocatalysis Using Metallic Cobalt Pyrite (CoS2) Micro- and Nanostructures, J. Am. Chem. Soc., 2014, 136 (28), pp 10053-100061, DOI: 10.1021/ia504099w
8. Matthew S. Faber, Mark A. Lukowski, Qi Ding, Nicolas S. Kaiser, and Song Jin; Earth-Abundant Metal Pyrites (FeS2, CoS2, NiS2, and Their Alloys) for Highly Efficient Hydrogen Evolution and Polysulfide Reduction, J. Phys. Chem. C, 2014, 118 (37), pp 21347-21356, DOI:10.1021/jp506288w
9. Junqiao Zhuo, Miguel Caban-Acevedo, Hangeng Liang, Leith Samad, Qi Ding, Yongping Fu, Meixian Li, and Song Jin, High-Performance Electrocatalysis for Hydrogen Evolution Reaction Using Se-Doped Pyrite-Phase Nickel Diphosphide Nanostructures, ACS Catal., 2015, 5 (11), pp 6355-6361, DOI: 10.1021/acscatal.5b01657
10. Miguel Cabán-Acevedo, Michael L. Stone, J. R. Schmidt, Joseph G. Thomas, Qi Ding, Hung-Chih Chang, Meng-Lin Tsai, Jr-Hau He, and Song Jin, Efficient hydrogen evolution catalysis using ternary pyrite-type cobalt phosphosulphide, Nature Materials, 2015, doi:10.1038/nmat4410
11. Tao Wu, Michael L Stone, Melinda J. Shearer, Matthew J. Stolt, Ilia A. Guzei, Robert J Hamers, Ruifeng Lu, Kaiming Deng, Song Jin, and Jordan R. Schmidt, Crystallographic Facet Dependence of the Hydrogen Evolution Reaction on CoPS: Theory and Experiments, ACS Catal., 2018, 8 (2), 1143–1152. DOI: 10.1021/acscatal.7b03167
12. Bo Song, Kai Li, Ying Yin, Tao Wu, Lianna Dang, Miguel Cabán-Acevedo, Jiecai Han, Tangling Gao, Xianjie Wang, Zhihua Zhang, J. R. Schmidt, Ping Xu, and Song Jin, Tuning Mixed Nickel Iron Phosphosulfide Nanosheet Electrocatalysts for Enhanced Hydrogen and Oxygen Evolution, ACS Catal., 2017, 7, pp 8549–8557, DOI: 10.1021/acscatal.7b02575
13. Hanfeng Liang, Fei Meng, Miguel Caban-Acevedo, Linsen Li, Audrey Forticaux, Lichen Xiu, Zhoucheng Wang, and Song Jin; Hydrothermal Continuous Flow Synthesis and Exfoliation of NiCo Layered Double Hydroxide Nanosheets for Enhanced Oxygen Evolution Catalysis, Nano Letters, 2015, pp. 1421-1427, DOI: 10.1021/nl504872s
14. Hanfeng Liang, Linsen Li, Fei Meng, Lianna Dang, Junqiao Zhuo, Audrey Forticaux, Zhoucheng Wang, and Song Jin, Porous Two-Dimensional Nanosheets Converted from Layered Double Hydroxides and Their Applications in Electrocatalytic Water Splitting, Chem. Mater., 2015, 27 (16), pp 5702-5711 DOI: 10.1021/acs.chemmater.5b02177
15. Lianna Dang, Hanfeng Liang, Junqiao Zhuo, Brandon K. Lamb, Hongyuan Sheng, Yang Yang, and Song Jin, Direct Synthesis and Anion Exchange of Non-Carbonate-Intercalated NiFe Layered Double Hydroxides and the Influence on Electrocatalysis, Chem. Mater. (2018) DOI: 10.1021/acs.chemmater.8b01334
16. Jamie Y. C. Chen, Lianna Dang, Hanfeng Liang, Wenli Bi, James B. Gerken, Song Jin, E. Ercan Alp, and Shannon S. Stahl, Operando Analysis of NiFe and Fe Oxyhydroxide Electrocatalysts for Water Oxidation: Detection of Fe4+ by Mössbauer Spectroscopy, J. Am. Chem. Soc., 2015, 137(48), pp 15090-15093, DOI: 10.1021/jacs.5b10699
17. Yang Yang, Lianna Dang, Melinda J. Shearer, Hongyuan Sheng, Wenjie Li, Jie Chen, Peng Xiao, Yunhuai Zhang, Robert J. Hamers and Song Jin, Highly Active Trimetallic NiFeCr Layered Double Hydroxide Electrocatalysts for Oxygen Evolution Reaction, Adv. Energy Mater., 2018, 1703189, DOI: 10.1002/aenm.201703189
18. Hongyuan Sheng, Eric D. Hermes, Xiaohua Yang, Diwen Ying, Aurora N. Janes, Wenjie Li, J. R. Schmidt, Song Jin. Electrocatalytic Production of H2O2 by Selective Oxygen Reduction Using Earth-Abundant Cobalt Pyrite (CoS2), ACS Catal., 2019, 9, 8433-8442. DOI: 10.1021/acscatal.9b02546
19. Wujun Liu, Lianna Dang, Zhuoran Xu, Han-Qing Yu, Song Jin, and George W. Huber, Electrochemical Oxidation of 5-Hydroxymethylfurfural with NiFe Layered Double Hydroxide (LDH) Nanosheet Catalysts, ACS Catal. 2018, 8 (6), 5533–5541. DOI: 10.1021/acscatal.8b01017
20. Qi Ding, Fei Meng, Caroline R. English, Miguel Caban-Acevedo, Melinda J. Shearer, Dong Liang, Andrew S. Daniel, Robert J Hamers, and Song Jin, Efficient Photoelectrochemical Hydrogen Generation Using Heterostructures of Si and Chemically Exfoliated Metallic MoS2, J. Am. Chem. Soc., 2014, 136(24), 8504-8507. DOI: 10.1021/ja5025673
21. Qi Ding, Jianyuan Zhai, Miguel Caban-Acevedo, Melind J. Shearer, Linsen Li, Hung-Chih Chang, Meng-Lin Tsai, Dewei Ma, Xingwang Zhang, Rober J. Hamers, Jr-Hau He, and Song Jin, Designing Efficient Solar-Driven Hydrogen Evolution Photocathodes Using Semitransparent MoQxCly (Q = S, Se) Catalysts on Si Micropyramids, Advanced Materials, 2015, 27, 6511-6518. DOI: 10.1002/adma.201501884
